Previous studies showed that sodium silicate was highly-active and recyclable with little deactivation as a heterogeneous catalyst in converting vegetable oils to biodiesel. Recently, microwave irradiation was used in the transesterification of vegetable oils to biodiesel, because of its high product selectivity, significant energy-saving and drastic reaction acceleration as compared with conventional heating systems. The Biomass Group of Xishuangbanna Tropical Botanical Garden (XTBG) conducted a study to examine the effect of sodium silicate on the transesterification of rapeseed oil and non-edible Jatropha oil under microwave irradiation conditions. Reaction process was examined and the catalyst was recycled to test its reusability. In order to make full use of the deactivated sodium silicate from biodiesel production, another objective of their work was to study hydrogen generation from glycerol in subcritical water with the reused sodium silicate and Ni catalyst. In addition, the effects of combination of Ni catalyst and sodium silicate on hydrogen production were also examined. Their study found that biodiesel yield of 95.8% was achieved from rapeseed oil at 400 W in 5 min. Biodiesel yield of 92.8% was achieved from Jatropha oil at 400 W in 5 min. The fourth cycled catalyst was utilized to gasify by-product glycerol at 350 °C. H2 yield of 82.8% (purity 73.6%) was achieved with the used and Ni catalysts. Therefore, microwave-assisted transesterification of vegetable oil with sodium silicate was an effective and economical method for the rapid production of biodiesel. Sodium silicate was fully used in biodiesel production and glycerol gasification, and the co-production process provided a novel green method in biodiesel production and glycerol utilization. The study entitled “Co-production of biodiesel and hydrogen from rapeseed and Jatropha oils with sodium silicate and Ni catalysts” has been published online in Applied Energy. 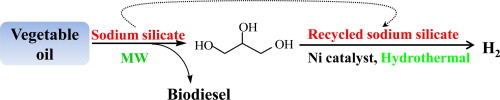
|

